
Institute of Chemistry, Academia Sinica, Taipei 11529, TAIWAN
Bridging Energy and Chemistry via Nanoarchitectonic Engineering at Atomic Scale
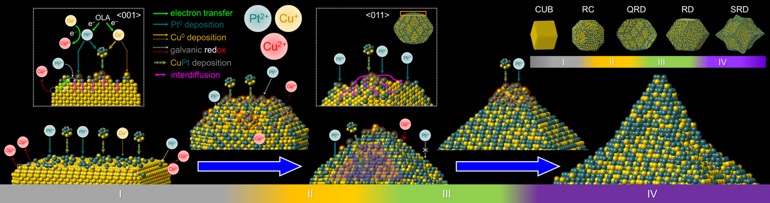
Building bimetallic surfaces on nanocrystals renders them superior catalysts for energy-conversion reactions by virtue of the electronic effect. In addition, integration of plasmonics and catalysis in bimetallic nanocrystals provides more benefits for the creation of novel catalyst systems. In this talk, selected stories about utilization of CuM bimetallic nanocatalysts for catalytic and photocatalytic energy-conversion reactions will be introduced.[1] All stories start by the synthesis of Cu nanocubes which serve as the templates for further surface engineering. On the Cu nanocubes, we reduce various kinds of noble metallic ions to form CuM surfaces with precise-controlled thickness less than 2 nm (7-8 atomic layers). In typical, template-based syntheses exhibit the coating shells with a consistent morphology to their mother nanotemplates via epitaxial growth. However, our atom-level surface modulation on the surfaces of Cu nanocubes exhibit unconventional morphological transformation after formation of CuM shells. Moreover, treating Cu-CuM core-shell nanocrystals with acid to remove Cu cores triggers further morphological transformation, leading to final nanocages in a specific shape. Those nanocages are eventually applied to electrocatalysis (oxygen reduction reaction, ORR) and photocatalytic synthetic reaction (4-nitrophenol reduction reaction). It turns out that these nanocages possess at least triply high mass activities than that of commercial catalysts. Apart from surface-coated shells, fabrication of CuM nanoframes on the Cu nanocubes via selective deposition of metallic ions will be also introduced. These nanoframes have 3D-open skeletons with highly exposed surface area and, more importantly, surface defects (i.e. adatoms and terraces).[2] The characteristics make CuM nanoframes excellent catalysts in both electro- and photocatalysis.
References
- Lyu, L.-M.; Kao, Y.-C.; Cullen, D. A.; Sneed, B. T.; Chuang, Y.-C.; Kuo, C.-H.*, Chem. Mater. 2017, 29, 5681.
- Ng, K. C.; Lin, F.-C.; Yang, P.-W.; Chuang, Y.-C.; Chang, C.-K.; Yeh, A.-H.; Kuo, C.-S.; Kao, C.-R.; Liu, C.-C.; Jeng, U.-S.; Huang, J.-S.; Kuo C.-H., Chem. Mater. 2018, 30, 204-213.


























